Nourella® is committed to increasing treatment results for all patients suffering from ageing skin. The primary focus of our research is focused on Dermal and Epidermal Hyperthrophy and clinically validated and proven Proteoglycan Replacement Therapy treatment.
Nourella® continues to partner with the world’s leading authorities on chronological and accelerated intrinsic and extrinsic skin ageing. Nourella®’s efficacy and safety have been documented in more than 75 scientific studies and clinical papers published in leading peer-review journals.
Nourella® has been tested in placebo, double-blind studies and shown to be side effect free. This is in contrast to other pharmaceuticals, which may cause short as well as long term side effects.
 Conclusion: Scientific literature suggests that the active ingredients of the Nourella® tablet and cream have several synergistic anti-ageing activities which substantiate their combined use in the Nourella® Active Skin Support System. In this long-term, controlled trial, the efficacy and safety data for combination therapy with topical RetileX-A® plus oral Vercilex® is provided. Before treatment, the participants with an average age of 48 had facial skin features similar to individuals in their 60s. By increasing skin thickness and elasticity, Nourella® combination therapy reversed skin characteristics to the values typical for persons in their 30s. This skin rejuvenating effect represents a significant skin age reversal of over 20 years. In line with objective measurements, the VAS of patient satisfaction with Nourella® therapy increased considerably (P<0.001). 80% of users reported a visible improvement in their skin. Importantly, treatment effect sizes and results of the combination therapy exceeded those of monotherapy with either Vercilex® or RetileX-A® in previous studies. In conclusion, Nourella® combination therapy is a preferred method in the prevention and the clinical management of skin ageing.
Conclusion: Scientific literature suggests that the active ingredients of the Nourella® tablet and cream have several synergistic anti-ageing activities which substantiate their combined use in the Nourella® Active Skin Support System. In this long-term, controlled trial, the efficacy and safety data for combination therapy with topical RetileX-A® plus oral Vercilex® is provided. Before treatment, the participants with an average age of 48 had facial skin features similar to individuals in their 60s. By increasing skin thickness and elasticity, Nourella® combination therapy reversed skin characteristics to the values typical for persons in their 30s. This skin rejuvenating effect represents a significant skin age reversal of over 20 years. In line with objective measurements, the VAS of patient satisfaction with Nourella® therapy increased considerably (P<0.001). 80% of users reported a visible improvement in their skin. Importantly, treatment effect sizes and results of the combination therapy exceeded those of monotherapy with either Vercilex® or RetileX-A® in previous studies. In conclusion, Nourella® combination therapy is a preferred method in the prevention and the clinical management of skin ageing.
 Conclusion: A wide range of cellular and molecular anti-ageing effects of retinol is compared with retinoic acid (tretinoin) in this meticulous clinical study. Although an unusually low concentration of retinol was compared with an unusually high strength of tretinoin, similar efficacy outcomes were observed. Both treatments induced significant epidermal thickening, proven by skin biopsy, after just 4 weeks of application. This study also provides strong evidence for the boosting effect of topical retinol and tretinoin on de-novo synthesis of procollagen and collagen I and III by skin cells. Face skin imaging revealed that applying retinol reduces wrinkling score by 64% at the cheeks and 39% around the eyes after 3 months of treatment. In conclusion, retinol is an equally effective but safer option that can replace tretinoin in the clinical prevention and treatment of chronological and accelerated skin ageing.
Conclusion: A wide range of cellular and molecular anti-ageing effects of retinol is compared with retinoic acid (tretinoin) in this meticulous clinical study. Although an unusually low concentration of retinol was compared with an unusually high strength of tretinoin, similar efficacy outcomes were observed. Both treatments induced significant epidermal thickening, proven by skin biopsy, after just 4 weeks of application. This study also provides strong evidence for the boosting effect of topical retinol and tretinoin on de-novo synthesis of procollagen and collagen I and III by skin cells. Face skin imaging revealed that applying retinol reduces wrinkling score by 64% at the cheeks and 39% around the eyes after 3 months of treatment. In conclusion, retinol is an equally effective but safer option that can replace tretinoin in the clinical prevention and treatment of chronological and accelerated skin ageing.

Conclusion: This clinical trial verified the efficacy and dose response of Nourella® tablet with Vercilex® against placebo in treating accelerated ageing of facial skin. The clinical assessments demonstrated that, only after 90 days of treatment, full-dose treatment improved facial skin thinning by 55% and skin laxity by 76% (P<0.01 compared to placebo for both). Pathological skin dryness was also treated efficiently. Interestingly, a low dosage of Vercilex® induced similar significant rejuvenating effects but at a lower magnitude. Clinical findings were confirmed by objective measurements with standard equipment showing dose-dependent improvements in skin thickness, elasticity and hydration. The majority of individuals who received the full dose of Vercilex® experienced positive changes in their skin 1-2 month(s) into the treatment. This research supports the efficacy of Vercilex® in the treatment of skin ageing and suggests that a continuous full-dose treatment is needed to produce optimal clinical improvements.

Conclusion: This trial was performed to evaluate the effect of nano-encapsulation on the anti-ageing efficacy of topical RP. Each subject received both the nano-encapsulated (RetileX-A®) and conventional RP. As such each patient was their own control. According to objective assessments, only RetileX-A® significantly improved skin thickness (+31%, P<0.01) and skin elasticity (+18%, P<0.01) after 3 months of therapy. The majority of participants recognised visible enhancements in the overall quality, smoothness and glow of their skin after using RetileX-A® (P<0.01) but not the conventional formulation. These findings suggest that nano-encapsulation has a substantial effect on bioavailability and bioactivity of topical RP and elevates its clinical efficacy. Thus, not all available retinoid formulations are equally effective.
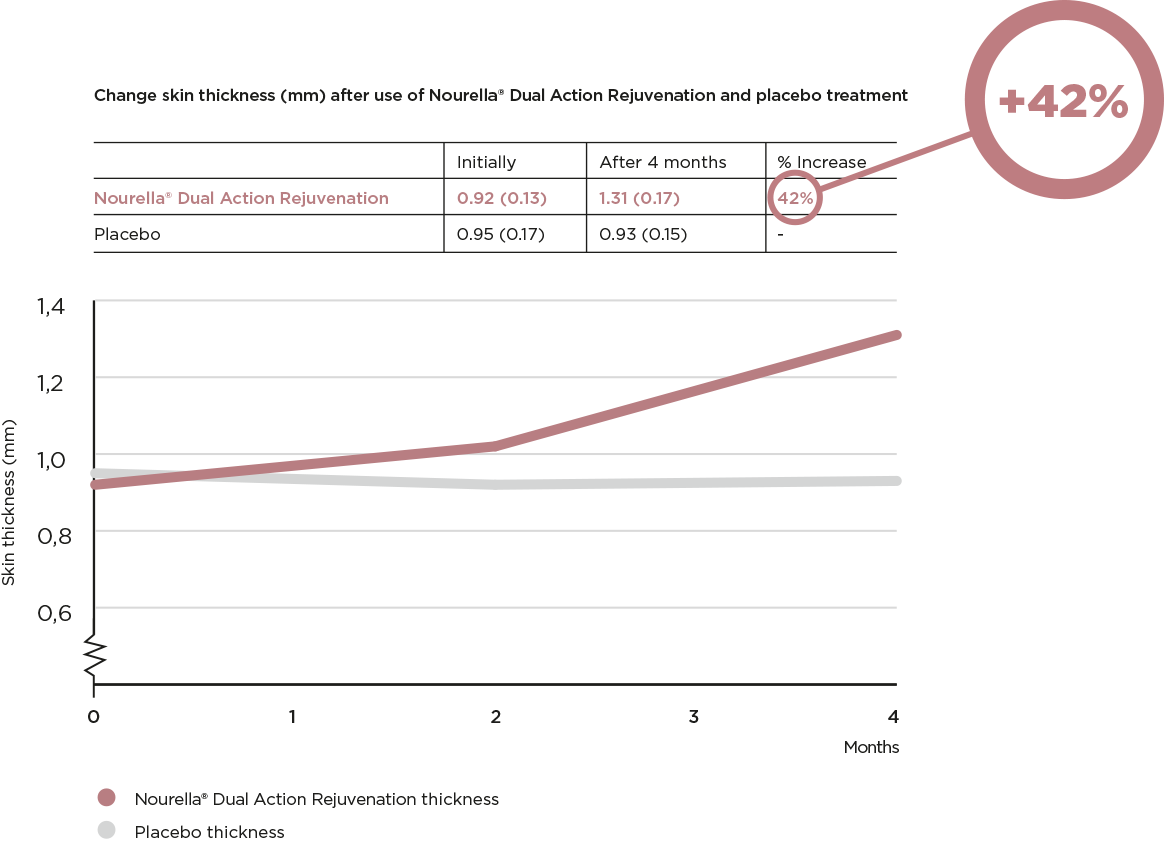
 Conclusion: The clinical effects of Nourella® tablet with Vercilex® on several subjective and objective outcomes were assessed in a randomised clinical trial. Just 4 months of Vercilex® therapy increased both thickness and elasticity of aged skin. This reached a significant level after 6 months (P<0.05 for both within- and between-group changes). All treated subjects in the Vercilex® group experienced positive changes. When asked to comment, 18 out of 20 subjects (90%) treated with Vercilex® reported an improvement in their skin quality compared with 5% in the placebo group. Optimal treatment satisfaction is reflected in a 6-unit increase in VAS score by Vercilex® compared to 0.2 units by placebo (P<0.001). This controlled trial provides reliable evidence for the efficacy of oral Vercilex® treatment in the management of skin ageing.
Conclusion: The clinical effects of Nourella® tablet with Vercilex® on several subjective and objective outcomes were assessed in a randomised clinical trial. Just 4 months of Vercilex® therapy increased both thickness and elasticity of aged skin. This reached a significant level after 6 months (P<0.05 for both within- and between-group changes). All treated subjects in the Vercilex® group experienced positive changes. When asked to comment, 18 out of 20 subjects (90%) treated with Vercilex® reported an improvement in their skin quality compared with 5% in the placebo group. Optimal treatment satisfaction is reflected in a 6-unit increase in VAS score by Vercilex® compared to 0.2 units by placebo (P<0.001). This controlled trial provides reliable evidence for the efficacy of oral Vercilex® treatment in the management of skin ageing.

Conclusion: In a follow-up evaluation, researchers set out to study if the rejuvenating effects of RP, observed in the previous trial, persist even after treatment discontinuation. The role of formulation technique was also examined by comparing nano-encapsulated (RetileX-A®) with non-conjugated RP forms. As much as 25% of therapeutic effects of RetileX-A® on skin thickness and 23% on skin elasticity were still present after one year of treatment cessation. On average, individuals treated with nano-encapsulated RetileX-A® had a significantly thicker (P<0.05) and more flexible (P<0.05) skin after a 12-month treatment-free period. When given a choice, the majority of participants could distinguish the forearm treated with RetileX-A® (P<0.05). In conclusion, the anti-ageing effects of RetileX-A® are significantly more lasting than the conventional unconjugated formulations.